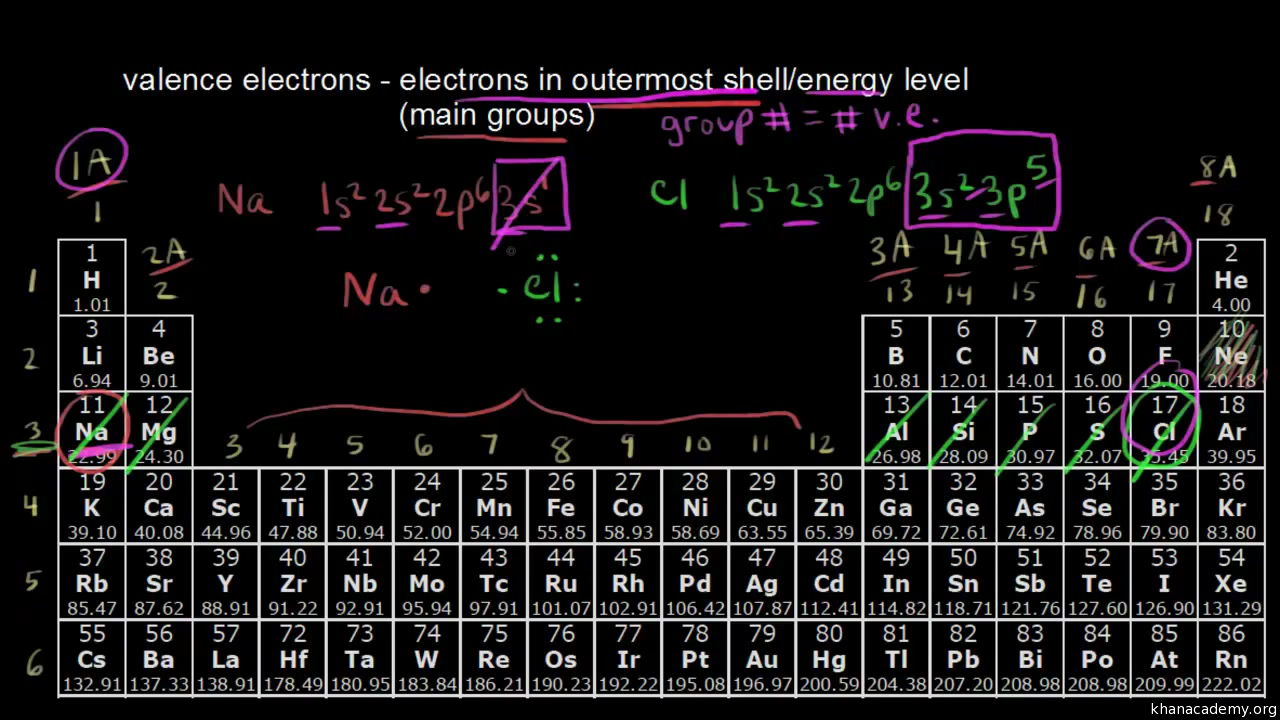
A sample of chlorine has two naturally occurring isotopes. The isotope Cl-35 (mass 35.0 amu) makes up 75.8% of the sample, and the isotope Cl-37 (mass = 37.0 amu) makes up the 24.3% of the sample. Its group number. Valence electrons are electrons located: In the outermost energy level of an atom. Therefore the group number is 7. Do note that in some periodic tables, they label the groups on a 1 - 18 system instead of a 1 - 8 system. If that's the case, the group number would be 17.
- Chlorine Group Number And Period
- 10 Facts About Chlorine
- What Is The Atomic Number Of Chlorine
- Chlorine Group Number On The Periodic Table
Element Chlorine (Cl), Group 17, Atomic Number 17, p-block, Mass 35.45. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images.

Chlorine Group Number And Period
Since it combines directly with nearly every element, chlorine is never found free in nature. Kingdom under fire heroes pc download. Chlorine was first produced by Carl Wilhelm Scheele, a Swedish chemist, when he combined the mineral pyrolusite (MnO2) with hydrochloric acid (HCl) in 1774. Although Scheele thought the gas produced in his experiment contained oxygen, Sir Humphry Davy proved in 1810 that it was actually a distinct element. Today, most chlorine is produced through the electrolysis of aqueous sodium chloride (NaCl).
Chlorine is commonly used as an antiseptic and is used to make drinking water safe and to treat swimming pools. Large amounts of chlorine are used in many industrial processes, such as in the production of paper products, plastics, dyes, textiles, medicines, antiseptics, insecticides, solvents and paints.
Two of the most familiar chlorine compounds are sodium chloride (NaCl) and hydrogen chloride (HCl). Sodium chloride, commonly known as table salt, is used to season food and in some industrial processes. Hydrogen chloride, when mixed with water (H2O), forms hydrochloric acid, a strong and commercially important acid. Other chlorine compounds include: chloroform (CHCl3), carbon tetrachloride (CCl4), potassium chloride (KCl), lithium chloride (LiCl), magnesium chloride (MgCl2) and chlorine dioxide (ClO2).
10 Facts About Chlorine
Chlorine is a very dangerous material. Liquid chlorine burns the skin and gaseous chlorine irritates the mucus membranes. Concentrations of the gas as low as 3.5 parts per million can be detected by smell while concentrations of 1000 parts per million can be fatal after a few deep breaths.
What Is The Atomic Number Of Chlorine

Chlorine Group Number On The Periodic Table
Bidayatul mujtahid indonesia pdf.

Comments are closed.